Abstract
Introduction: Persistent cytopenia in the post-HCT setting remains a challenge in some recipients despite good engraftment of donor cells. PLX-R18 is a placental-derived mesenchymal-like cell product, expanded ex vivo in a 3-dimentional environment according to Good Manufacturing Practices. PLX-R18 cells secrete a large array of hematopoietic factors including stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), interleukin-6 and interleukin-11, which promote regeneration, maturation, and differentiation of hematopoietic cells in bone marrow and stimulate their migration to peripheral blood. Reported herein are the final encouraging safety and efficacy results from a phase 1 study (NCT03002519).
Methods: 21 patients > 18 years who experienced incomplete hematopoietic recovery at least 3 months post-HCT, were enrolled into the study. Eligibility criteria were defined as meeting at least one of the following: neutrophil count (ANC) < 1000/μL, platelet (PLT) < 50,000/μL or hemoglobin (HGB) < 8 g/dL. Patients had no evidence of other causes for cytopenia such as an active infection or malignancy or severe acute or chronic Graft vs. Host Disease. For allogeneic (allo) HCT, complete, stable donor chimerism was required. Safety of PLX-R18 therapy was the primary objective. PLX-R18 was administered via multiple IM injection in 2 treatment sessions one week apart. Patients were treated in 3 cohorts: 1 million cells/kg (n=3); 2 million cells/kg (n=6); and 4 million cells/kg (n=12).
Safety: 19 of 21 treated patients (90%) received an allo-HCT; for 5 patients (24%) this was a repeat HCT. The median time from HCT to PLX-R18 administration was ~8 months (236 days). Acute lymphocytic leukemia (ALL) was the most common underlying malignancy (33%) followed by acute myeloid leukemia (14%) and myelofibrosis (14%). The most common adverse events (AEs) were local injection-site reactions (pain), observed in 85.7% of patients, and were transient and of mild to moderate severity. No clinically meaningful changes were observed in vital signs, ECG and blood counts, liver and kidney function or coagulation laboratory results. A transient and reversible elevation in inflammatory markers, high sensitivity C-reactive protein (hsCRP) and complement factor 5a (C5a), were observed on the day following PLX-R18 administration and returned to normal levels thereafter. One patient developed severe febrile neutropenia two days after the second infusion (PLX-R18 4 million cells/kg) which was considered related to PLX-R18 and resolved 2 days later. Four AEs were associated with a fatal outcome (1 sudden death, 2 events of sepsis and 1 event of ALL recurrence); none were considered by the Investigator, or the Sponsor, as related to PLX-R18. One relapse was reported, an ALL patient who relapsed 296 days following study treatment and passed away 2 weeks later. No other events of relapse or recurrence were reported.
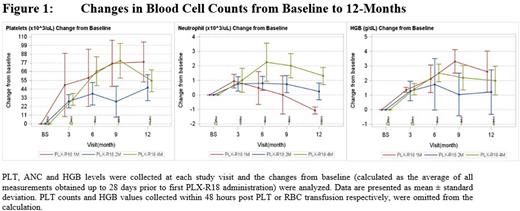
Efficacy: For all PLX-R18 doses, a positive mean change from baseline in PLT and HGB was observed starting as early as 1 month after PLX-R18 administration and lasting up to 12 months following treatment (p<0.05). Positive mean change from baseline in ANC was observed in all doses as early as one month, peaking at 6 months for the 4 million cells/kg dose (p=0.062). Among patients who received blood transfusions at any time during follow up, a meaningful reduction in the mean number of transfused units per month, for both PLT and RBC transfusions, was observed as early as 3 months following PLX-R18 treatment and lasted throughout the 12-month follow up period. For PLT, the mean monthly number of transfused units dropped from 5.09 at baseline to 0.55 at 12 months (p<0.05); for RBC, from 2.91 at baseline to 0.00 at 12 months (p=0.0005).
Conclusion: PLX-R18 therapy appears to be safe and well tolerated and shows promise in improving incomplete hematopoietic recovery after HCT. Further clinical studies are warranted to optimize the efficacy of PLX-R18 in treatment of cytopenia in the post-HCT setting and expand the potential to other marrow failure disorders as well as cytopenia associated with certain treatment modalities such as CAR-T therapy.
Disclosures
Lazarus:Pluristem: Consultancy, Honoraria. McGuirk:Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; In8bio, Inc.: Other: IIT Clinical Trial; CRISPR Therapeutics: Consultancy; Sana: Honoraria; Orca Bio: Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Speakers Bureau; Nextar: Consultancy, Honoraria. Zuckerman:Cellect Biotechnology: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Orgenesis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BioSight Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Metheny:Gamida: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau. Rowley:ReAlta Life Sciences: Consultancy; SIRPant Immunotherapeutics: Consultancy. Avni:MSD: Membership on an entity's Board of Directors or advisory committees. Sheleg:Pluristem Ltd.: Current Employment. Rothenstein:Pluristem Ltd.: Current Employment. Halevy:Pluristem Ltd.: Current Employment. Rowe:Biosight: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.